Sylc, Bioactive Glass: The Benefits are Intrinsic
By John C. Comisi, DDS, MAGD
By John C. Comisi, DDS, MAGD
Introduction
Stephen Mann has described the principles of biomineralization in 2001 as “how living organisms secrete inorganic minerals in an organized manner with exceptional physical properties by virtue of finely controlled microstructure, morphology and hierarchal organization of the minerals and accompanying organic material.”1 That is, that the body is able to take selected inorganic materials when presented in the proper way and in the proper quantities and use them to essentially reconstruct solid structure, such as tooth structure.
When Professor Larry Hench, at the University of Florida, first developed a novel compound of calcium sodium phosphosilicate (CSPS) designed based on a a class of materials known as bioactive glass, the purpose was so it could used in the development of bone regeneration materials.2 Over time a particular composition of this substance, was derived and later given the trade named Novamin®, also referred to as 45S5, and was later used in dental applications. Novamin® is an amorphous melt-derived glass compound, identical to Bioglass® which is the best known bioactive glass, and contains only calcium, sodium, phosphate and silica, which was found to be very beneficial in the reduction of dental hypersensitivity.
From Medicine to Dentistry
How is something that was derived for bone regeneration translated to dental applications? Well, until the development of these bioglasses all biomaterials were made as inert as possible for use in the human body so as to not be problematic to the system. However, once these bioglass materials were found to be able to create a direct chemical bond to bone tissue3, the utilization of inert materials began to wane since these materials were able to bridge a gap when a section of bone was missing and firmly secure the sections together. This discovery allowed researchers to change direction and focus on developing mechanisms that could be able to positively interact with the body and to allow healing to occur.
When investigations were undertaken to better understand the mechanism of action it was discovered that these CSPS materials go through a series of reactions at the surface of the material to release ions into the surrounding structures and that the surface of the CSPS material would be changed in composition and structure.4 The interaction at the interface of the CSPS and bone structure showed that the CSPS particles became negatively charged which would facilitate the absorption of proteins, calcium and phosphates that would over the course of the interaction form hydroxycarbonate apatite (HCA), which would then become a bonding interface between the bone and the implant containing the CSPS. This reaction has been demonstrated time and time again in study after study. Using a bioactive glass can and will enable biomineralization to occur. Another interesting finding of the may study on CSPS is that its reactivity has been shown to be somewhat antibacterial against oral bacteria and transient anti-inflammatory properties 5,6,7.
Which leads us to the application of CSPS, namely Novamin® in dentistry. Dental hypersensitivity is based on hydrodynamics. When dentinal tubules are open, fluid flow is allowed which then causes an excitation of the odontoblasts and thereby the dental pulp. This opening of the dentinal tubules occurs when the cementum of the root surface or the smear layer are removed from the root of the tooth either by abrasion or erosion. The number open tubules and the diameter of the tubules will determine the type and intensity of the hypersensitivity.
Chemical agents such as potassium nitrate or potassium chloride, which are commonly used, penetrate into the dentinal tubules and depolarize the nerve synapse; reducing the sensitivity by preventing conduction of pain impulses.8 This unfortunately can often take a long period of time for the patient to perceive a benefit. Other chemical agents have been used as well, including, potassium oxalate, ferric oxalate, and strontium chloride to physically occlude the dentin tubules, reducing the fluid flow and reducing sensitivity. Although effective, the symptoms often reoccur due to physical abrasion of the root surfaces, acid challenges in the mouth and also the degradation of the actual materials themselves over time.
The use of Novamin® containing products, enables the bioactive glass particles to bind to the exposed dentin tubules and due to the size of the particles, they can also physically fill the open tubules. From the earlier studies on, it has been demonstrated that Novamin® can rapidly occlude the exposed tubules and form a protective layer on the dentin surface9. Novamin®, when exposed to an aqueous environment (water or saliva), immediately releases sodium ions and increases the pH of the local environment creating. This acidity creates a more rapid release and then a precipitation of the other ions present (calcium and phosphates) to form an amorphous calcium phosphate layer within minutes of its application, which then creates an hydroxycarbonate apatite (HCA) layer. Early studies10 found that even in the absence of external calcium and phosphates, a calcium HCA layer will be formed.
We must also remember that there is silica contained in the CSPC family and the silica in Novamin® has been shown to physically occlude the dentinal tubules. Additionally, the presence of soluble silica promotes the precipitation of HCA, even in the presence of HCA inhibitors11. In fact, it has been demonstrated that the interactions of small three-member silica chains create an optimal environment forming nucleating sites for HCA formation12.
In order for effective binding and occluding of the dentinal tubules the Novamin® particles must be able to not only release ions of sufficient concentrations of time, but they must also be able to remain intimately in contact with the dentin over time. Studies13,14 have demonstrated that when this bioglass develops the negative surface charge when it initially reacts with the tooth structure and saliva, it also binds to the Type 1 collagen fibers present in the dental tubules.
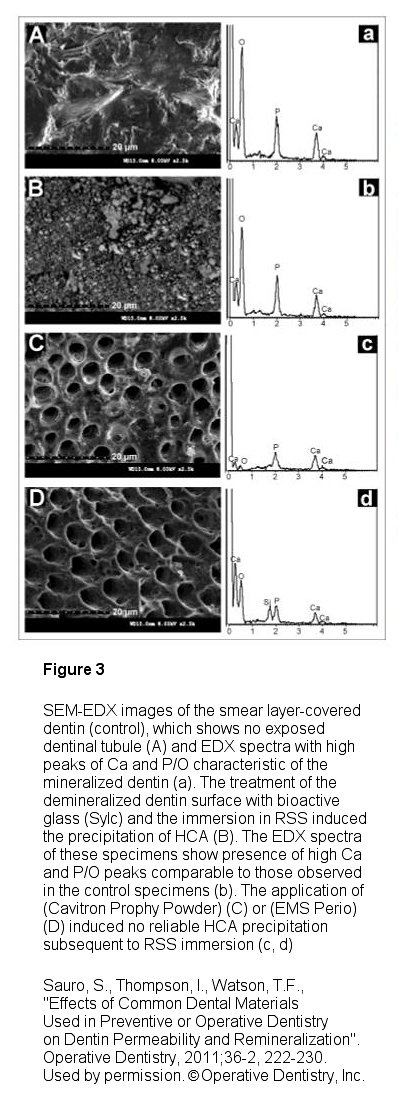
Sylc, (Figure 1) which is a commercialized therapeutic powder that is composed of 100% Novamin® bioactive glass, provides the various benefits previously described in powder form that is used in an air polishing instrument delivery system. The powder is available in its original polishing formulation and now in a stain removing formula. When used in this methodology, it has been found to be a very effective agent in polishing and stain removal as well as for desensitization. Interestingly it has been shown by Sauro, Thompson and Watson15 that Sylc can also be used for operative dentistry as an air-cutting powder in contactless minimally invasive dentistry air abrasion instrumentation (such as an AquaCare unit, Figure 2) as a substitute to alumina in non-mechanical cavity preparation. Their work illustrates that “its clinical use has many advantages, including reduced pain experienced by patients and selective removal of carious dentins and rounded internal cavity angle preparations that minimizes stress concentration". Additionally, it was found that Sylc is a suitable method to remineralize dentin and reduce permeability as compared to other air cutting powders used and is indicated as a beneficial prior to traditional resin-bonding procedures to prevent further demineralization and even remineralize the hybrid layer within the resin-dentin interface when used during traditional dental bonding procedures (Figure 3).
There has also been the introduction recently of glycine-based powders, with lower abrasiveness compared bicarbonate based prophy powders. While these powders are softer then alumina, and sodium bicarbonate powders and are designed to be used subgingivally during supra and subgingival deplaquing, the efficiency of these powders for dentin desensitization, however, is inferior to the benefits of HCA formation we have described being provided by the bioglass found in Sylc16. So it must be considered that the use of a softer material, such as glycine, with the intent of not creating sensitivity, instead may be creating opportunities for greater sensitivity since the dentinal tubules are not being occluded. Further there is no formation of HCA as there is with Sylc that could otherwise help decrease sensitivity and protect the surfaces of the roots of teeth.
Conclusion
The utilization of bioactive glass has been used in medicine for several decades and the availably of these types of agents have been available in dentistry as well. When the benefits of the biomineralization were finally realized in medicine the movement toward the use of bioactive materials was embraced. In dentistry, the conversation around bioactivity has been called by some as just a fad or a buzz to sell products. However, it is indisputable that the utilization of bioactive materials can and will be a major leap forward in what we do in dentistry.
The benefits of Novamin® in Sylc powder has been demonstrated for stain removal, polishing, desensitization of the hypersensitive tooth structure and even for the cutting of tooth structure with an air abrasion unit. The ability of Sylc to create hydroxyapatite when in contact with tooth structure, saliva and dentin fluid is a benefit that should be considered in everyday clinical practice to overcome the limitations found in our current typical dentin bonding procedures. Using Sylc in air abrasion units in cavity preparation and/or prior to using resin-bonding procedures can potentially improve the bonding by reducing demineralization15 via the known defensive enzymatic process.17,18.
1. Stephen Mann, Biomineralization - Principles and Concepts in Bioinorganic Materials Chemistry. Oxford University Press. 2001.
2. Hench, L.L., “Biomaterials”, Science 1980; 208:826-831.
3. Greenspan, D.C., “Novamin® and tooth sensitivity - An overview”. J Clin Dent 2010;21[Spec. Iss.]:61-65.
4. Hench, L.L., Paschall, H. A., “Direct chemical bond of bioactive glass-ceramic materials to bone and muscle”. J Biomed Mater Res 1973;7:25-42.
5. Stoor, P., Söderling, E., Salonen J.L., “Antimicrobial effects of a bioactive galls passion on oral microorganisms.” Acts Odontol Scand 1998;56:161-165.
6. Allan, I., Newman, H., “Antibacterials activity of particulate bioglass paste on oral microorganisms.” Biomaterials 2001;22:1683-1687.
7. Rechtenwald, J.E., Minter, R. M., Rosenberg, J.J., Gaines, G.C., Lee, S., Moldawer, L.L., “Bioglass attenuates a proinflammatory response in mouse peritoneal endotoxicosis”. Shock 2002;17:135-138.
8. Schiff, T., Bonta, Y., Proskin, H. M., Pertrone, M., Volpe, A.R., “Desensitizing efficacy of a new dentifrice containing 5.0% potassium nitrate and 0.454% stannous fluoride”. Am J Dent 2000;13:111-115.
9. Litkowski, L. J., Hack, G.D., Sheaffer, H.B., Greenspan, D. C., “Occlusio of dentin tubules by 45S5 bioglass”. In: Bioceramics 10, Sedel L., Rey, C., eds., Proceedings of the 10th International Symposium on Ceramics in Medicine, Paris, France, October, 1997.
10. Hench, L.L., Andersson, Ö., “Bioactive glasses. In: Introduction to Bioceramics, Hench, L.L., Wilson, J., eds., Singapore, World Scientific, pp. 41-62, 1993.
11. Damen, J.J., ten Cate, J.M., “Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation”. J Dent Res 1992;71:453-457.
12. Shari, N. Anseau, M., “Cyclic silicate active site and sterichemical match for apatite nucleation on pseudowollastonite bioceramic-bone interfaces”. Biomaterials 2005;26:5763-5770.
13. Zhong, J.P., LaTorre, G.P., Hench, L.L., “The kinetics of bioactive ceramics Part VII: Binding to collagen to hydroxyapatite and bioactive glass. In: Bioceramics 7, Andersson O.H., Yli-Urpo, A., eds., Proceedings of the 7th International Symposium on Ceramics in Medicine, Turku, Finland, July 1994.
14. Orèfice, R., Hench, L.L., Brennan, A., “Evaluation of the interactions between collagen and the surface of a bioactive glass during in vitro test”. J Biomed Mater Res 2009;90:114-120.
15. Sauro, S., Thompson, I., Watson, T.F., “Effects of Common Dental Materials Used in Preventive or Operative Dentistry on Dentin Permeability and Remineralization”. Operative Dentistry, 2011;36-2, 222-230.
16. Sauro, S., Watson, T.F., Thompson, I., “Utramorphology and dentine permeability changes induced by prophylactic procedures on exposed dentinal tubules in middle dentine”.Medicina Oral Patología Oral Y Cirugia Bucal 2011 Nov 1;16 (7):e1022-30.
17. Mazzoni, A., L. Tjaderhane, V. Checchi, R. Di Lenarda, T. Salo, F. R. Tay, D. H. Pashley, and L. Breschi. "Role of Dentin MMPs in Caries Progression and Bond Stability." Journal of Dental Research 94.2 (2014): 241-51.
18. Comisi, J.C., “The ‘Reservoir Restorative’ Revolution”, Dentistry Today, Volume 35 No.7 Page 126